Systemic Chemotherapy
By Paul T. Finger, MD
Description
Chemotherapy commonly refers to the use of drugs to treat cancer of the eye, lids and orbit. Some of these drugs are also used to treat benign ocular tumors caused by inflammation.
Though this review tends to focus on the side-effects of chemotherapy, it is important to note that most patients are able to tolerate treatment, are helped by chemotherapy, and go on to enjoy their lives.
Clearly the use of chemotherapeutic drugs have cured patients and extended lives. Chemotherapy also offers a method to control inflammatory diseases and provide an alternative or adjunct to surgery and radiation.
How Chemotherapy Drugs Are Given
Systemic chemotherapy drugs can be infused into a patient’s vein (intravenous – IV) or by mouth (per oral – PO). These methods can expose the entire body to significant doses of chemotherapy.
In ophthalmology, we also give chemotherapy topically (ointment or drops) onto the eye. This places the drug on top of the tumor or affected area and limits the possibility of systemic (whole body) side effects. In ophthalmology, certain drugs are also given by periocular injection. The physician’s choice of delivery method depends on the type of chemotherapy drug and the reason why it is given.
Why Chemotherapy Drugs Are Given
Chemotherapy drugs are typically prescribed to attack or destroy a patient’s tumor or cancer. They can also be used to change a patient’s immune system. They can be used alone or in combination with radiation and/or surgery. In general, systemic chemotherapy is given when a patient needs a systemic (whole body) therapy, or when the risks of chemotherapy are less than those associated with radiation or surgery.
Side Effects & Complications
All drugs can have side-effects (unwanted effects). It is important to ask your doctor or nurse about the potential risks (side-effects) and benefits associated with your chemotherapy. This is important because each drug can have slightly different types of side-effects.
There are common side-effects associated with many chemotherapy drugs. Since these reactions may or may not be associated with your chemotherapy, you may want to ask if they will apply to your case.
A partial list of side effects includes: allergies, bone marrow depression, hair loss, mouth and throat problems, nausea and vomiting, sexual and reproductive problems, and skin changes.
Allergic Reactions
Allergic reactions can occur after taking almost any drug (including chemotherapy). They can occur immediately or can be delayed. If severe, the drug will be discontinued. If mild, allergy treatments – e.g. suppression agents (typically more drugs) can be used to proceed with chemotherapy.
Common allergic symptoms include:
- Rashes on the skin
- Swelling
- Shortness of breath
- Rapid heart beat
Rare allergic symptoms include:
- Decreased blood pressure
- Shock
- Kidney failure
Since most chemotherapy is given in a clinic or hospital, patients are typically watched for reactions after drugs are administered. In these controlled environments, prompt treatment is administered and allergic reactions are treated.
When at home after treatment, patients can notice late allergic reactions. If you notice skin rashes, weakness while standing or sitting, progressive swelling or any unusual changes you should contact your physician immediately.
Bone Marrow Suppression
Bone marrow depression (suppression) is a side effect caused by certain chemotherapy drugs. When it occurs, it typically happens 7-10 days after the chemotherapy is started and usually recovers after 3 weeks. There are biotherapy medications that can speed bone marrow recovery.
Blood cells and other blood components are made in the bone marrow. Therefore, bone marrow depression results in decreased numbers of white blood cells, red blood cells, and platelets.
When the body loses white blood cells (leukopenia), it is a greater risk for infection.
When the body loses red blood cells (anemia), it can be associated with fatigue.
When the body loses platelets (thrombocytopenia), the blood has trouble clotting and the patient is at greater risk for bruising and bleeding.
Symptoms suggestive of bone marrow depression?
- Fever or chills
- Fatigue
- Bruising or Bleeding
If you have fever, chills, fatigue, bruising or bleeding call your doctor immediately. In most cases your physician can give you medicines to treat bone marrow depression. These drugs can treat infections and compensate for low levels of white blood cells, red blood cells, and platelets.
Hair Loss – Alopecia
Hair loss (alopecia) is a common side effect of chemotherapy, but not all chemotherapy drugs cause hair loss.
Not all hair loss is total. It can affect the head or all parts of the body. It can be gradual. Typically hair loss does not happen immediately. It starts several weeks after the first chemotherapy, then hair can thin or come out in clumps. Some people also experience scalp sensitivity.
It makes sense to use a mild shampoo, a soft hair brush, and low heat when drying your hair. Avoid dyes, perms and hair relaxers. In general, a short hair cut looks fuller. If you don’t cover your head, you may need to use sun screen on your scalp.
It is normal to feel angry or depressed when you lose your hair. You should speak with your doctor or nurse about your feelings. There may be a support group or mental health professional who can help you. But remember, hair loss is usually a temporary side-effect. The hair will grow back.
Hats and Wigs
Most sources say you should shop for a wig before you lose most of your hair. That way you can match your usual style and hair color. Consider getting a wig with tape-tab materials called “stickies.” This will allow you to comb and style the wig without worrying about sliding. Most sources also suggest you buy two wigs so you can have one on while the other is being cleaned.
Check to see if your insurance company will cover its cost. If not, it is a tax-deductible expense (keep your receipt). Have the establishment write, “hair prosthesis as prescribed by doctor” on the receipt.
When buying a hat, make sure it will block the sun from hitting your scalp. Women also prefer to wear hats or turbans when at home. Don’t forget your sunglasses.
Mouth and Throat Problems
Chemotherapy can cause sores, red areas or white patches to form on the mouth and throat. This is particularly true for systemic methotrexate and 5-fluorouracil.
Since mouth and throat care are known to improve, stabilize or prevent progression of mouth sores, patients should call their doctor if mouth sores or soreness occurs.
Typical Mouth Care Instructions May Include:
- Regular, gentle cleaning of your teeth or dentures.
- Also, rinse your mouth with a mixture:
- 1/2 teaspoon of salt in 8 ounces of water or
- 1/2 teaspoon of sodium bicarbonate (baking soda) in 8 ounces of water.
- This should be done after every meal and at bedtime.
- Avoid alcohol containing mouthwash.
- Avoid acidic or spicy foods.
- Avoid drinking alcohol and smoking.
Your doctor may have different methods of mouth care. So contact your doctor if you develop mouth sores, white patches, mouth pain or difficulty swallowing.
Nausea and Vomiting
Nausea and vomiting are the body’s defenses against eating too much and its mechanism to get rid of toxic food and poisons. During vomiting, the muscles that normally push food down the gastrointestinal tract, reverse direction and send back the partially digested material.
Nausea and vomiting may occur within the first hour of chemotherapy and can last up to a week. Most of the time nausea goes away shortly after these drugs are given.
In severe cases, anti-nausea medications may be necessary.
That is because the vomiting patient may lose too much fluid and necessary minerals. When a patient is at risk of dehydration and blood chemical (electrolyte) imbalance, your doctor or nurse can prescribe medication to help you prevent or lessen nausea.
Other causes of nausea that cancer patients may be experience include stress, radiation of the brain, and anesthetics (associated with surgery).
Treatment of Nausea and Vomiting
The primary treatment for nausea is anti-nausea drugs. Since they work best taken before chemotherapy, discuss this with your doctor before your next treatment (particularly if you were nauseated after your last treatment).
Other methods include: acupressure wrist bands which are available at most drug stores, fasting (do not eat) for a couple of hours before your treatment, try to keep your eyes open, and slowly deep-breathing through your nose.
If you are already nauseated from chemotherapy, do not eat a large meal or drink carbonated beverages. You may try to eat crackers, pretzels, or dry toast. Avoid foods with a lot of residue, salads, high fiber cereals or fatty and fried foods.
Sometimes, right after chemotherapy patients are only able to suck on ice chips, sip ice water, or mild herbal tea. Once patients are feeling better, they may benefit from chicken soup.
If nausea and vomiting persist, call your doctor. Some patients need to receive intravenous (IV) fluids and electrolytes (minerals).
Sex and Reproduction
Chemotherapy-related sexual and reproductive problems can occur in both men and women.
Since certain chemotherapy drugs may have harmful effects on an unborn child, genetic counseling should be made available to the chemotherapy patient in order to discuss the effects of drug therapy on current and future pregnancies.
Studies & Research
- Wilson MW, Czechonska G, Finger PT, Rausen A, Hooper ME, Haik BG. Chemotherapy for Eye Cancer Survey of Ophthalmology 45:416-444, 2001.
- Calabresi P, Chabner BA. Chemotherapy of Neoplastic Diseases: Introduction. In: Goodman and Gilman’s: The Pharmacological Basis of Therapeutics. Tenth Edition Hardman JG, Limbird LE, Gilman AG (eds.) McGraw-Hill, New York, 2001, Section IX, pp.1381-1388.
- Chabner BA, Ryan DP, Paz-Ares L, Garcia-Carbonero R, Calabresi P. Antineoplastic Agents. In: Goodman and Gilman’s: The Pharmacological Basis of Therapeutics. Tenth Edition Hardman JG, Limbird LE, Gilman AG (eds.) McGraw-Hill, New York, 2001, Chapter 52, pp.1389-1460.

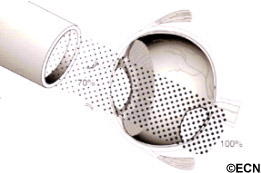
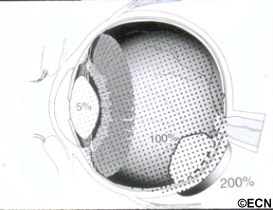
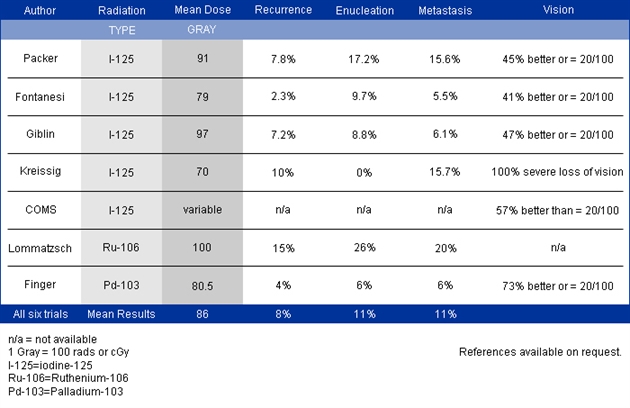
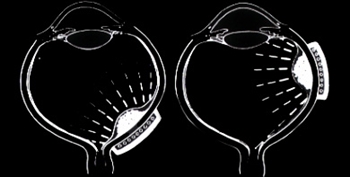 Subsequently, Yousef reported a total lack of radiation maculopathy after palladium-103 plaque therapy for iris and iridociliary tumors.21 These clinical observations clearly linked radiation dose to fovea to the incidence of radiation maculopathy.
Subsequently, Yousef reported a total lack of radiation maculopathy after palladium-103 plaque therapy for iris and iridociliary tumors.21 These clinical observations clearly linked radiation dose to fovea to the incidence of radiation maculopathy.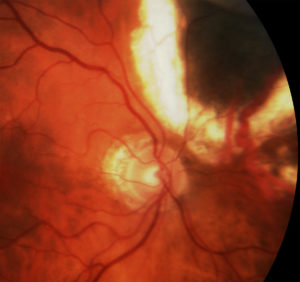 However, in 2005, I published the first evidence that laser demarcation of the posterior 180º around the tumor was effective for preventing or delaying radiation maculopathy.25
However, in 2005, I published the first evidence that laser demarcation of the posterior 180º around the tumor was effective for preventing or delaying radiation maculopathy.25
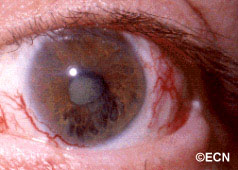
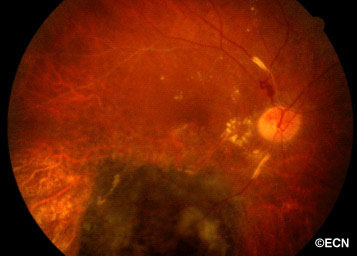 Above, this is an intraocular photograph of a patient treated with ophthalmic plaque radiation who later developed optic nerve neovascularization, intraretinal microangiopathy, chorioretinal atrophy, a ghost vessel, and perivascular sheathing. The dark tumor on the bottom of the photograph was inactive (sterilized).
Above, this is an intraocular photograph of a patient treated with ophthalmic plaque radiation who later developed optic nerve neovascularization, intraretinal microangiopathy, chorioretinal atrophy, a ghost vessel, and perivascular sheathing. The dark tumor on the bottom of the photograph was inactive (sterilized).