Two Extensive ECF-Sponsored Studies Presented at the AAOOP Annual Meeting
The New York Eye Cancer Center and the Eye Cancer Foundation were quite actively represented at the 2017 American Association of Ophthalmic Oncologists and Pathologists (AAOOP) Annual Meeting. The meeting was held on Friday, November 10, 2017 at the Hampton Inn & Suites Convention Center, located in the vibrant city of New Orleans, Louisiana, and was attended by Dr. Paul Finger as well as notable ECF-ICO Fellowship alumni, Dr. Sonal Chaugule, Dr. Ekatrina Semenova, and Dr. Abhilasha Maheshwari.
At the conference, Dr. Chaugule gave an oral presentation titled Adjuvant intravitreal triamcinolone acetate (ITA) for radiation maculopathy (RM) recalcitrant to high-dose intravitreal bevacizumab. This research was supported by the Eye Cancer Foundation and conducted at the New York Eye Cancer Center, where Dr. Chaugule worked alongside Dr. Richard Kaplan (ophthalmologist) and Dr. Paul T. Finger. She is pictured speaking on this paper at AAOOP below:
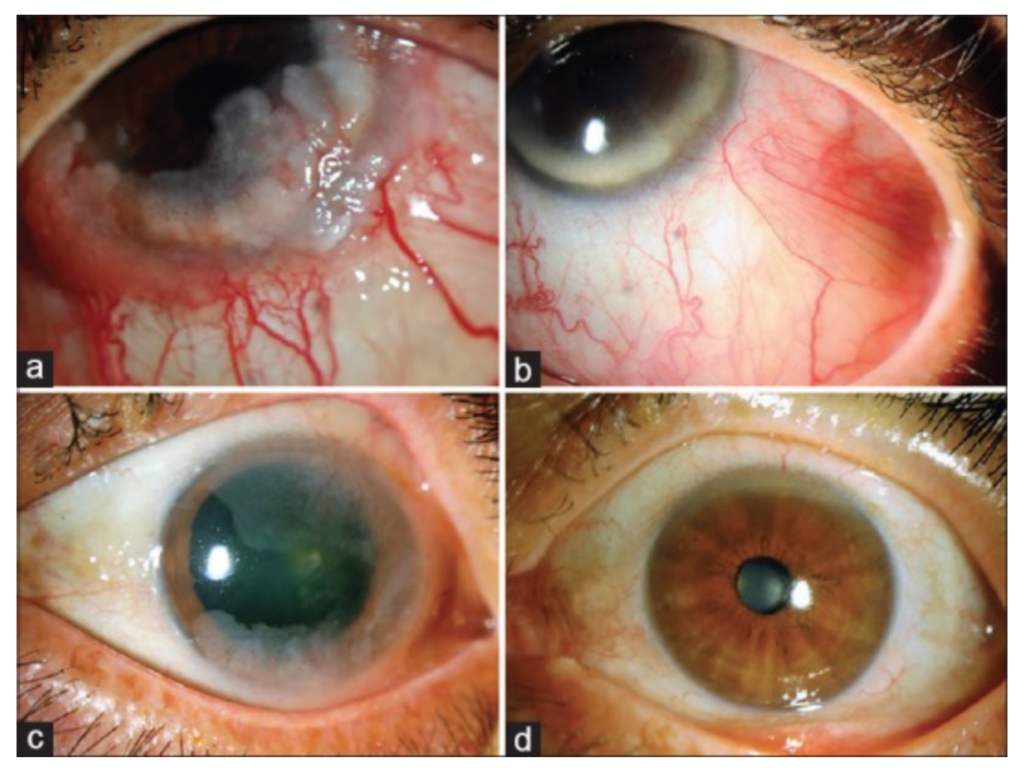
Now, what are ITA, RM, and Bevacizumab? Often, patients undergoing eye plaque radiation in order to treat their cancerous tumor can be subject to vision-impairing radiation side-effects, or radiation maculopathy (RM), as a result of treatment. Intravitreal anti-VEGF therapy (which is otherwise commonly used to treat macular degeneration) such as Bevacizumab (Avastin), Lucentis, and Eyelea, are used to prolong the effects of radiation maculopathy. Itravitreal triamcinolone acetate (ITA) is a steroid used in conjunction with this anti-VEGF therapy to treat swelling that occurs in the affected eye, called macular edema.
The paper aims to evaluate the effects of using ITA for the treatment of RM in patients with choroidal melanoma after plaque radiotherapy. Eight choroidal melanoma patients undergoing this treatment were studied, having ITA treatment at 4-16 week intervals in addition to continued injections of Avastin. Results found that after starting ITA, vision was stable or improved for patients, leading to the conclusion that ITA can be used as a supplement to decrease macular edema (swelling) and preserve vision in choroidal melanoma patients with RM.
The evaluation of ITA steroids as valuable treatment for RM is not to be underestimated. It provides a new treatment option for patients experiencing loss of vision due to radiation therapy, patients whose loss of vision can no longer be controlled with maximum, standard anti-VEGF therapy. To read more on the findings of this paper and its effect on eye cancer patients, click here. And to read this paper in full, published in the British Journal of Ophthalmology, click here.
Dr. Abhilasha Maheshwari had separately presented ECF-supported research — a 12-year study evaluating patients with slotted, low energy photon eye plaque radiation therapy. The purpose? To measure the efficacy of this treatment for eye cancer patients, especially those who have tumors located near, touching, or surrounding the optic disc (a critical area that allows for vision) were treated. Forty six patients of these eye cancer patients were treated with eye plaque radiation therapy, using seeds of the chemical isotope Palladium-103 to radiate the affected eye. Over the next 12 years, these patients were monitored for any changes to tumor thickness, visual acuity, and whether or not the cancer had reoccured or metastasized. Results found that the local control rate (i.e, total tumor destruction) was 95.6%, and lead to the conclusion that Slotted Eye Plaque Radiation Therapy is indeed an efficient method of treatment for eye cancer patients. To read the paper, published in the American Journal of Ophthalmology, click here.
But the AAO updates do not end here! Stay tuned for upcoming information on even more presentations at AAO 2017 by ECF alumni.



 From the summer of 2016 to 2017, Dr. Sonal Chaugule gained a wealth of knowledge as an Eye Cancer Foundation (ECF) fellow under the tutelage of Dr. Paul T. Finger at the NYECC. From shadowing Dr. Finger’s surgeries at the New York Eye and Ear Infirmary, to speaking at the Second Eye Cancer Working Day in March 2017, Dr. Chaugule has since returned to India, using the knowledge she has gained as tools to continue in the footsteps of her mentors. Her efforts manifest in
From the summer of 2016 to 2017, Dr. Sonal Chaugule gained a wealth of knowledge as an Eye Cancer Foundation (ECF) fellow under the tutelage of Dr. Paul T. Finger at the NYECC. From shadowing Dr. Finger’s surgeries at the New York Eye and Ear Infirmary, to speaking at the Second Eye Cancer Working Day in March 2017, Dr. Chaugule has since returned to India, using the knowledge she has gained as tools to continue in the footsteps of her mentors. Her efforts manifest in