By Paul T. Finger, MD, FACS
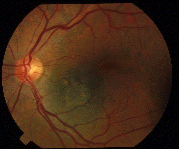
In ophthalmology we are blessed with many well-defined clinical indications where the potential benefits outweigh the risks of treatment; where we clearly help our patients. This is not always the case in treatment of “small choroidal melanomas.” [1-3] At first blush, the choice appears simple, loss of vision (in one eye) versus death (due to metastatic melanoma). A closer look reveals controversy’s in the diagnostic criteria used for small choroidal melanomas, that treatment may or may not risk loss of central vision and that observed tumor-growth may be the best predictor of malignancy.
Diagnosing Small Choroidal Melanomas
In the past, eyes were enucleated with the diagnosis of choroidal melanoma only to find simulating or benign lesions on histopathology.[4] The advent and evolution of modern diagnostic techniques such as indirect ophthalmoscopy, ultrasonography, photography, angiography and subspecialty centers have improved our diagnostic ability to detect small changes in tumor size and patterns of leakage.
Tumor-specific characteristics have been found to carry diagnostic value. [5][6] For example, a choroidal tumor with orange pigment on its surface, thickness of greater than 2 mm and subretinal fluid is very likely to be a small malignant choroidal melanoma. Mainly using these criteria, The Collaborative Ocular Melanoma Study (COMS) physicians were reported to correctly diagnose medium and large-sized choroidal melanomas in 99.6% of cases.[7] Documented tumor growth [over a relatively short period of time (typically months)] is commonly used to differentiate a suspicious choroidal nevus from a small choroidal melanoma.[8] Observation for growth is used because there currently exists no safe and effective method to obtain a biopsy (cytopathologic proof) of tumors that small (less than 10 x 10 wide x 3 mm thick).
The Case for “Observation as Treatment”
Many eye cancer specialists watch small choroidal melanomas for evidence of tumor-growth prior to treatment. This is because current belief is that “small choroidal melanomas” carry a low risk (6-10%) for metastases and that all current treatments risk severe vision loss.[9-11] Current observation of small choroidal melanomas is justified by the concept that “tumor-growth demonstrates malignancy.” In practice, documented tumor growth both reassures the doctor that the tumor is malignant and reassures the patient that treatment is indicated. Specifically, the risk of treatment-related loss of vision is more than offset by a reduction in the probability of metastasis and tumor-induced vision loss.
This is particularly true for patients with small choroidal melanomas close to the fovea, monocular or systemically ill patients. In these cases, serial observation may allow for years of useful vision prior to treatment. For all patients, the case for observation of small melanoma growth has been governed by the potential benefit of vision preservation (in the affected eye).
However, the risk of vision loss in radiation treated eyes has recently diminished. In 2006, Finger discovered and others have found that anti-VEGF medications can preserve vision in eyes affected by radiation maculopathy and optic neuropathy. These studies suggest that radiation damage may no longer inevitably lead to vision loss and changes the balance between treatment and observation.
The Case for Immediate Treatment
Once an eye cancer specialist is convinced that a tumor is a malignant (albeit small) choroidal melanoma, treatment becomes the most reasonable choice. In support of this approach, one can cite Packard’s and the Collaborative Ocular Melanoma Study’s (COMS) findings that increased tumor size (specifically largest tumor diameter – LTD) was associated with an increased risk of metastatic death.[10,11] Therefore, it is reasonable to assume that waiting for documentation of malignant melanoma growth increases (albeit marginally) a patient’s risk for metastases.
This is no surprise. Increasing tumor size had been associated with an increased incidence of metastases in cancers of the eye, breast, lung, and colon, and cutaneous melanoma. Throughout medical oncology there exists a fundamental understanding that early treatment of cancer saves lives. This concept has led to the development of national strategies aimed at early cancer detection.
I could find no cancer monitoring or treatment programs that promote observation of cancer growth when treatment is available. Similarly, ophthalmic oncologists would not promote observation of small malignant eyelid or conjunctival melanomas.
Currently, all patients with small choroidal melanoma can be offered eye and vision-sparing treatments. Particularly, if one uses the tumor’s apex as the prescription point for plaque radiation therapy (as recommended by the American Brachytherapy Society), small choroidal melanomas require particularly small treatment volumes that should be associated with less resulting vision loss.[12]
Recent evidence suggests that should radiation retinopathy and optic neuropathy occur, it is treatable with anti-VEGF agents (eg. Avastin or Lucentis).13 Lastly, immediate treatment will prevent the tumor enlargement associated increased risk of metastatic melanoma.
Continued Observation Once Definitive Growth Has Been Documented
Very few eye cancer specialists would recommend continued observation once definitive tumor-growth has been documented. Choroidal nevi grow, but typically exhibit small changes over years of observation. Relatively rapid (e.g. months) and measurable tumor growth is consistent with malignancy. Therefore as currently practiced widely, documented rapid tumor enlargement indicates that a “suspicious choroidal nevus” is actually a malignant choroidal melanoma, and will (by continued enlargement and/or secondary retinal detachment) cause loss of vision. Once a small melanoma has grown, treatment offers the best chance for preservation of both life and vision in the affected eye.
Hope from the Future
Current research offers the potential to aid in the diagnosis of small choroidal melanoma. Onken, Harbour et al are exploring the use of molecular and genetic markers to assess a tumors metastatic potential.14 Genetic studies suggest that monosomy 3, together with other genetic markers may define a tumor’s metastatic potential. Similarly, physiologic-radiographic imaging (such as PET/CT) may offer the potential to assess a tumors metabolic activity and metastatic potential.15-18
Expect new methods to address treatment-related complications, decrease morbidity and preserve functional vision.19 Lastly, we will see earlier detection of metastatic disease.16,17 When metastatic disease is found, local therapies will be largely abandoned in favor of systemic treatment.
Doing the Least Harm
This editorial explores the controversy’s surrounding treatment of small choroidal melanomas. “Observation as treatment” for suspected small choroidal melanomas offers the patient time (without the risk of treatment-related vision loss) at the risk (small increase in the probability) of death from metastatic choroidal melanoma. This is why, despite our advances in the diagnosis and treatment of these tumors, there exists considerable controversy “among experts” about what characteristics differentiate small choroidal melanomas from indeterminate choroidal tumors and when treatment is warranted.2,8,20
Current practice dictates that eye cancer specialists continue to perform clinical assessments, classify small choroidal tumors and discuss the potential risks and benefits of observation, biopsy and treatment with each patient. Keep in mind that your doctors recommendation will be influenced by his or her experience with treatment-related risk for vision loss versus that due to metastatic melanoma. Further, physicians will determine the patient’s ability to understand what has been presented and recommend an approach to do the “least” harm.
Summary
Until better methods of differentiation are available, “Observation as treatment” will continue to be the standard of care for benign and suspicious choroidal nevi, as well as most small indeterminate choroidal tumors. Treatment will be recommended for small malignant choroidal melanomas, particularly if those tumors are documented to grow.
References
- Barr CC, Sipperley JO, Nicholson DH. Small melanomas of the choroid. Arch Ophthalmol 1978;96(9):1580-2.
- Char DH. The management of small choroidal melanomas. Surv Ophthalmol 1978;22(6):377-86.
- The Collaborative Ocular Melanoma Study Group. Mortality in patients with small choroidal melanoma. COMS report no. 4. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol 1997;115(7):886-93.
- Chang M, Zimmerman LE, McLean I. The persisting pseudomelanoma problem. Arch Ophthalmol 1984;102(5):726-7.
- Augsburger JJ, Schroeder RP, Territo C, et al. Clinical parameters predictive of enlargement of melanocytic choroidal lesions. Br J Ophthalmol 1989;73(11): 911-7.
- The Collaborative Ocular Melanoma Study Group. Factors predictive of growth and treatment of small choroidal melanoma: COMS Report No. 5. The Collaborative Ocular Melanoma Study Group.” Arch Ophthalmol 1997;115(12): 1537-44.
- Collaborative Ocular Melanoma Study Group: Accuracy of diagnosis of choroidal melanomas in the Collaborative Ocular Melanoma Study. COMS Report No. 1. Arch Ophthalmol 108:1268-1273, 1990.
- Augsburger JJ. Is observation really appropriate for small choroidal melanomas. Trans Am Ophthalmol Soc 1993;91:147-68; discussion 169-75.
- Finger PT. “Radiation therapy for choroidal melanoma.” Surv Ophthalmol 1997;42(3):215-32.
- Packard R B. “Pattern of mortality in choroidal malignant melanoma.” Br J Ophthalmol 1980;64(8):565-75.
- The Collaborative Ocular Melanoma Study Group. Mortality in patients with small choroidal melanoma. COMS report no. 4. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol 1997;115(7):886-93.
- Nag, S, Quivey JM, Earle JD, Followill D, Fontanesi J, Finger PT. The American Brachytherapy Society recommendations for brachytherapy of uveal melanomas. Int J Radiat Oncol Biol Phys 2003;56(2):544-55.
- Finger PT. Radiation retinopathy is treatable with anti-vascular endothelial growth factor bevacizumab (Avastin). Int J Radiat Oncol Biol Phys 2008;70:974-7.
- Onken MD, Worley, LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res 2004;64(20):7205-9.
- Reddy S, Kurli M, Tena L, Finger PT Whole body PET/CT imaging: Detection of Choroidal Melanoma. Br J Ophthalmol 2005;89(10):1265-9.
- Finger PT, Kurli M, Reddy S, Tena LB, Pavlick AC (2005) Whole body PET/CT for initial staging of choroidal melanoma. Br J Ophthalmol 2005;89(10):1270-4.
- Finger PT, Kurli M (2005) Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol 89;730-8.
- 18-Fluorine-labelled 2-deoxy-2-fluoro-D-glucose positron emission tomography/computed tomography standardised uptake values: a non-invasive biomarker for the risk of metastasis from choroidal melanoma. Br. J. Ophthalmol 2006;90(10):1263-6.
- Kurli M, Reddy S, Tena LB, Pavlick AC, Finger PT (2005) Whole body positron emission tomography / computed tomography (PET/CT) staging of metastastic choroidal melanoma. Am J Ophthalmol 2005;140(2):193-9.
- Murray TG (1997). Small choroidal melanoma. Arch Ophthalmol 115(12): 1577-8.
Related Links
- WOC: Bio-informatics Can Solve the Mystery of Treatment of Small Choroidal Melanomas – Download the PDF Here
- Search PubMed for Small Choroidal Melanoma: To Treat or Not to Treat, That is the Question!
- Search Google for Small Choroidal Melanoma: To Treat or Not to Treat, That is the Question!









