Castle Biosciences, Inc. recently launched a new test to help doctors better asses the risk of uveal melanoma metastasis.
Uveal melanoma ranks as the most common eye cancer in adults. About half of patients diagnosed with uveal melanoma develop metastatic disease, primarily in the liver.
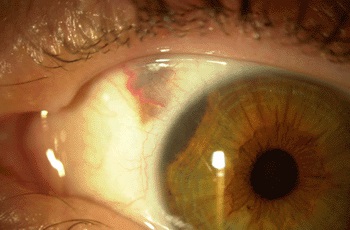
Researchers have found gene expression levels of PRAME (Preferentially Expressed Antigen in Melanoma) correlate with an increased risk of metastasis in patients diagnosed with uveal melanoma. The newly developed gene expression profile (GEP) test measures levels of PRAME.
“Our research shows that the expression of PRAME above a specific threshold in uveal melanoma is a strong predictor of increased metastatic risk in patients with a Class 1 uveal melanoma who otherwise would be assumed to be at low risk,” University of Miami School of Medicine Dr. J. William Harbour, said. “By combining results of the gene expression profile and PRAME tests, we believe we can enhance the accuracy of metastatic risk stratification in patients with uveal melanoma.”
The test is designed to be used in conjunction with DecisionDx-UM, the company’s primary uveal melanoma test.
“DecisionDx-UM is the standard of care to identify patients whose eye tumors are likely to spread and become deadly,” President and CEO of Castle Biosciences Derek Maetzold said. “We believe the DecisionDx-PRAME test used in conjunction with the results of the DecisionDx-UM test can enable further precision of a patient’s predicted risk for metastasis and help guide physicians and patients to the most appropriate follow-up care regimens.”
In a study published last March in Clinical Cancer Research, researchers found that PRAME could distinguish between GEP Class 1 tumors that would metastasize versus those that would not. Through the study, researchers were able to determine a threshold to signify positivity in uveal melanoma tumors. Tumors that metastasized in the study were all positive for PRAME expression according to this threshold.
Castle Biosciences went on to perform technical validation of the threshold in a 958 patient sample study. In addition, researchers found patients with high metastatic risk Class 2 tumors who were positive for PRAME expression may be at higher risk of earlier metastasis compared to patients with Class 2 tumors that were PRAME negative.
Dr. Paul Finger said that while more study is necessary, the new test shows promise.
“Though we all look forward to an independent GEP and PRAME validation study, these tests are promising developments for measurement of the risk for uveal melanoma metastasis,”he said. “Early treatment and therefore early detection is key to prolonging life. Therefore this new PRAME-test should help doctors better define an individual patients risk for choroidal melanoma metastasis.”









