A drug thrust into the limelight by Jimmy Carter shows promise for treating melanoma of the eyelid.
Keytruda (pembrolizumab) gained FDA approval for limited treatment of metastatic melanomas in late 2014. The agency gave its blessing for front-line treatment in patients with unresectable or metastatic melanoma on Jan. 7 of this year.
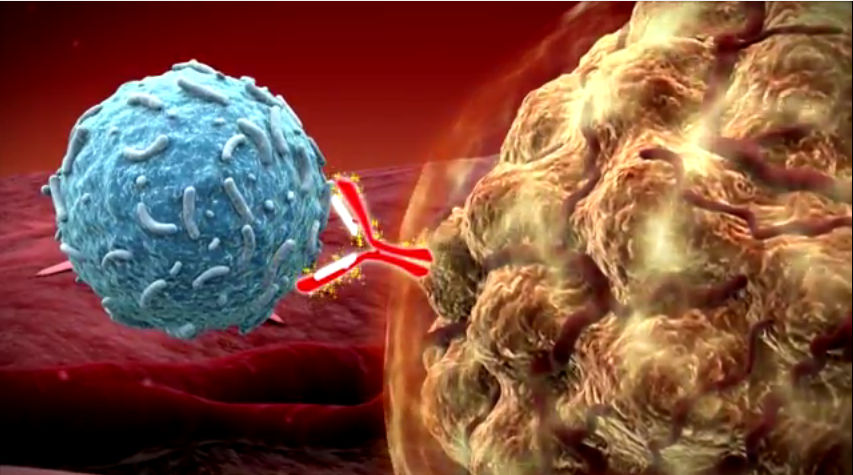
The drug is a form of immunotherapy and works by unleashing the body’s immune system to attack cancerous melanin cells. If the cancer is not caught early, it can spread deep into the skin and spread to other organs. At that point, chemotherapy becomes generally ineffective. Testing has shown Keytruda can drastically increase survival rates for advanced skin cancer.
Keytruda is what is known as a PD-1 inhibitor. T-lymphocyte cells are the body’s primary cancer fighter. Tumors express proteins called PD-L1 and PD-L2. These “program death” proteins lure T-lymphocyte cells to bind to them. Eventually, this results in T-cell exhaustion and reduces their ability to fight the cancer. Keytruda works by targeting the PD-1 receptors, allowing the body’s immune system fight the cancer cells. UConn doctors say they have had great success using Keytruda, according to Dr. Upendra P. Hegde, an associate professor in the Department of Medicine.
“Keytruda is the first PD-1 inhibitor drug that is allowing us to shrink the melanoma tumors in up to 35 percent of our UConn Health patients, and we are continuing to see more progress over time.”
A study reported in the Wall Street Journal last spring compared Keytruda to an older immunotherapy called Yervoy. The study revealed a significant increase in overall survival rates.
“The study…tested Keytruda against Yervoy in more than 830 patients whose melanoma had spread to other parts of the body. About one-third of patients received Keytruda once every two weeks, one-third received it every three weeks, and the remaining one-third received the standard four cycles of Yervoy. One year after the start of treatment, 74% of patients who received Keytruda every two weeks and 68% of those who received it every three weeks were still alive, compared with 58% for those who received Yervoy. Measured another way, Keytruda reduced the risk of death by 31% to 37% versus Yervoy, according to the results, which were published online Sunday by the New England Journal of Medicine and presented at the annual meeting of the American Association for Cancer Research in Philadelphia.”
A similar drug approved for melanoma treatment has shown similar promise. Opdivo (nivolumab) works in the same way as Keytruda, blocking the PD-1 receptors. A study presented at the 2015 Society for Melanoma Research Congress showed the 2-year overall survival rate with frontline nivolumab was 57.7%.
Jimmy Carter underwent treatment for his melanoma with Keytruda. He was diagnosed with an aggressive form of skin cancer that had spread to his brain and liver. In December, the former president announced he was cancer-free, putting the cancer-fighting drug in the national spotlight.
Learn more about metastatic tumors here.